Data from the Phase 2 Clinical Trial of CX11/VCT220 in China Presented at ADA 2025
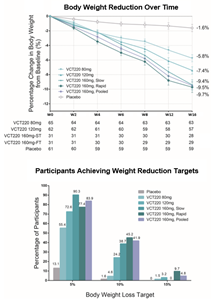
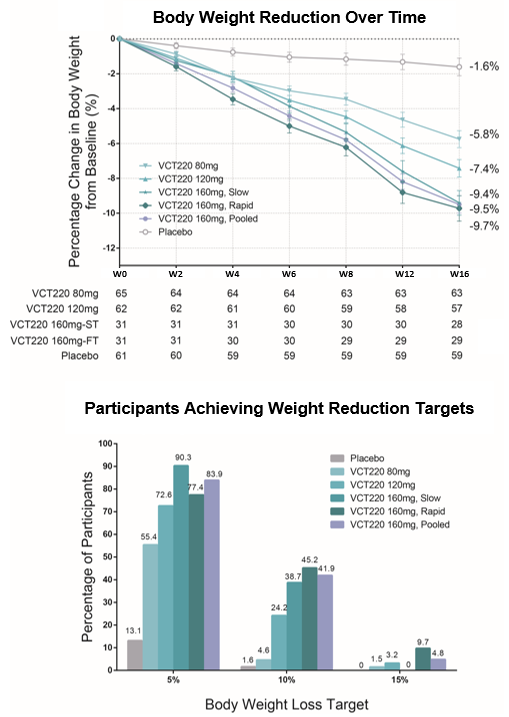
- Patients treated with CX11 (also designated as VCT220) achieved a 9.7% and 9.4% decrease in body weight from baseline after 16 weeks (p≤0.001) in the 160 mg fast and slow titration cohorts, respectively.
- Results suggest a favorable tolerability profile, with no drug-induced liver injury observed.
BERKELEY HEIGHTS, N.J. and SHANGHAI, June 23, 2025 (GLOBE NEWSWIRE) -- Corxel Pharmaceuticals Limited (CORXEL), a clinical-stage biopharmaceutical company dedicated to developing innovative therapies for patients with cardiometabolic conditions around the world, in collaboration with its partner, Chengdu Vincentage Pharma Co., Ltd. (Vincentage), announced the positive China Phase 2 clinical results for its oral small molecule once-daily glucagon-like peptide-1 receptor agonist (GLP-1 RA) CX11/VCT220, presented via poster presentation at the American Diabetes Association’s (ADA) 85th Scientific Sessions. The abstract will also be published online on the Diabetes® journal’s website.
The Phase 2 randomized, double-blind, placebo-controlled clinical trial was conducted by Vincentage to investigate the efficacy, safety and tolerability of CX11/VCT220 in nondiabetic adults with obese or overweight conditions in China.
The China Phase 2 trial evaluated 250 adults with a BMI greater than or equal to 28 kg/m2, or between 24 kg/m2 and 28 kg/m2 accompanied by at least one obesity-related comorbidity, randomized 3:1 to 80 mg, 120 mg, or 160 mg cohorts to receive CX11/VCT220 or matching placebo. The 160 mg cohort was further divided (1:1) into fast or slow titration groups. The primary endpoint was percentage change from baseline to week 16 in body weight (BW).
Results Summary:
At baseline, mean BW was 91.76 kg, mean BMI was 32.03 kg/m2, and 93.6% of participants had a BMI greater than or equal to 28 kg/m2. At week 16, the mean percentage change from baseline in BW ranged from −5.8% to −9.7% across the CX11/VCT220 dose cohorts and was −1.6% in the placebo group. Weight reduction was significantly greater at all CX11/VCT220 doses compared to placebo. A weight reduction of at least 5% by week 16 occurred in 55% to 90% of the participants who received CX11/VCT220, as compared to 13% who received placebo. (Figure). Treatment with CX11/VCT220 was associated with improvement in all weight-related and cardiometabolic metrics that were measured, including liver enzymes (ALT, AST) and blood pressure. Most reported adverse events were GI-related and mild to moderate (95.8%) in severity. No liver toxicity safety signal was identified. No drug-related serious adverse events were reported.
The data presentation at ADA marks the first time CX11/VCT220's clinical results are showcased on a global stage.
“We are pleased with the data presented at ADA that demonstrated robust efficacy results and favorable tolerability of CX11/VCT220,” said Dr. Bo Liang, Senior Vice President, Head of Clinical Development – Metabolic at CORXEL. “With the solid scientific foundation built by Vincentage, we have initiated the Phase 2 trial in the U.S. for CX11 with the first patient enrolled last week. We believe the Phase 2 trial in the U.S. has the potential to improve upon the weight reduction and tolerability results observed in the Phase 2 clinical trial of CX11/VCT220 in China.”
“We are excited to see CX11/VCT220 gain international attention,” said Dr. Ben Li, Chief Executive Officer of Vincentage. “The Phase 3 registrational trial in China was initiated in November 2024 and we look forward to supporting CORXEL in their global development efforts.”
About CX11
CX11 is an investigational oral small molecule GLP-1 RA designed to address cardiometabolic conditions, with a primary focus on obese and overweight patients. With global ex-China rights acquired by CORXEL from Vincentage in December 2024, CX11 aims to overcome limitations of current injectable GLP-1 RAs by offering convenient, once-daily oral administration, and weight reduction comparable to injectable GLP-1 RAs. CX11 presents favorable tolerability results, scalability and accessibility as a small molecule product candidate. Clinical data from Vincentage’s Phase 2 trial in China demonstrated competitive weight loss with a favorable safety and tolerability profile. CX11 is currently advancing into a Phase 2 trial in the U.S. conducted by CORXEL and through a Phase 3 registrational trial in China conducted by Vincentage, positioning it as a potentially leading oral therapy for management of obese and overweight conditions.
About CORXEL
CORXEL is a clinical-stage biopharmaceutical company dedicated to developing innovative therapies for patients with cardiometabolic conditions around the world. CORXEL is led by an experienced management team that has a strong track record of identifying, in-licensing and developing attractive clinical product candidates directed at validated targets with proven mechanisms of action (MoAs). CORXEL’s diverse portfolio of clinical-stage product candidates has the potential to redefine treatment standards and address key limitations of current therapies for multiple cardiometabolic indications. CORXEL is developing selective small molecule compounds across the cardiometabolic spectrum with the lead product candidate CX11, an oral GLP-1 receptor agonist (GLP-1 RA) for obese and overweight patients, JX10, a thrombolytic and anti-inflammatory agent for acute ischemic stroke (AIS) and JX09, a highly selective aldosterone synthase inhibitor (ASI) for hypertension.
For further information about CORXEL, please visit www.corxelbio.com
Contact:
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e5ddca94-9e35-420d-93da-1f4a84bab329

Data from the Phase 2 Clinical Trial of CX11/VCT220 in China
Corxel Pharmaceuticals Limited, in collaboration with its partner, Vincentage Pharma Co., Ltd., today announced the positive China Phase 2 clinical results for its oral small molecule once-daily glucagon-like peptide-1 receptor agonist (GLP-1 RA) CX11/VCT220, presented via poster presentation at the American Diabetes Association’s (ADA) 85th Scientific Sessions.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.